Authors: Noel SP, Courtney HS, Jennings JA, Baumgartner JD, Haggard WO
Title: Evaluation of Antibiotic-Loaded Chitosan Films as a Localized Drug Delivery System
Institution: University of Memphis, Biomedical Engineering, Memphis, TN
Purpose: The purpose of this study was to evaluate the efficacy of a chitosan film as a carrier for antibiotics in a drug delivery system. Chitosan films were evaluated based on drug release, mechanical integrity, and activity of eluates.
Methods: Evaluation of Antibiotic-Loaded Chitosan Films as a Localized Drug Delivery System.
- Authors:
Noel SP, Courtney HS, Jennings JA, Baumgartner JD, Haggard WO
Purpose: The purpose of this study was to evaluate the efficacy of a chitosan film as a carrier for
antibiotics in a drug delivery system. Chitosan films were evaluated based on drug release, mechanical
integrity, and activity of eluates. - Methods:
Film preparation: Chitosan films were prepared by dissolving 1.5 grams of chitosan (AgraTech), 80% deacetylation, into 98.5 milliters (ml) of 1 (V/V) % of a weak acid solvent mixture. 100 milligrams (mg) of antibiotic were dissolved into the chitosan solution. The chitosan solution was cast into glass Petri dishes (50 ml per dish) and placed into a 37°C convection oven for a drying period of 12 hours. After drying, the films were neutralized in sodium hydroxide solution and washed three times in distilled water. Films were sterilized by ethylene oxide gas and low-dose gamma irradiation.
- Elution tests:
5/8" discs were punched from the chitosan films and weighed in order to obtain a theoretical amount of antibiotic present. The discs were placed into 50 ml of Dulbecco's Modified Eagle's Media (DMEM) in glass vials. The vials were gently agitated at 37°C in an incubator. One ml aliquots were taken at designated timepoints. The aliquots were tested for antibiotic concentration using a fluorescence polarization immunoassay technique via TDxFLx instrument (Abbott Labs) for amikacin and vancomycin.
- Activity tests:
Activity of the antibiotic-containing eluates was tested by performing a series of turbidity assays. The assays allowed for quantitative measurement of antibiotic activity against S. aureus. 200 ?l of each eluate was added to tubes containing 1.8 ml of Mueller-Hinton II broth with a 50 ?g/ml CaCl2 supplement. Tubes were inoculated with 20 ?l of S. aureus and placed in a 37°C incubator for 24 hours. Absorbance measurements were taken and recorded after incubation at a wavelength of 530 nm (A530) on a spectrophotometer. Blanks were used to zero the spectrophotometer (1.8 ml of MHII broth and 200 ?l of PBS) and a percent inhibition determination was made when comparing eluate absorbance measurements to positive control absorbance measurements.
- Mechanical Testing:
Samples were subjected to tensile testing to determine the modulus of elasticity.
Results: Significant elution of both amikacin and vancomycin occurred after the first hour in solution.Amikacin release was determined to be 50.63 ?g/ml. The 48 hour release of amikacin was found to be 4.7 ?g/ml dropping to 0.6 ?g/ml at 72 hours (table 1). Vancomycin released from the chitosan film was found to be 83.45 ?g/ml after one hour. The release concentrations dropped significantly at hour 3 to 19.35 ?g/ml reaching a final release concentration of 6.13 ?g/ml after 72 hours (figure1, table 1). Turbidity assays performed with amikacin eluates showed that the antibiotics being released were active against S. aureus. These eluates were diluted 1:10 in the MHII broth. The eluates containing amikacin had an inhibition of 88.48% when compared to the positive control after 1 hour. After 72 hours, the inhibition rose to 98.71%. The modulus of elasticity for these chitosan films was found to be 3156.4 mega-pascals (MPa), 1950.5 MPa, and 859.9 MPa for the various solvent mixtures.
Discussion and Conclusion: Chitosan is a well?studied biocompatible material that can be used to deliver therapeutic agents in vivo. The localized delivery of antibiotics is an emerging area of study that could offer the benefit of high local concentrations of drug while avoiding the problems associated with whole? body dosing. This study characterized the in vitro release of different antibiotics eluting from a chitosan film. Both amikacin and vancomycin eluted from the films in a desirable manner and the eluates were found to be active against S. aureus. Vancomycin is the current gold?standard treatment method for methicillin?resistant S. aureus. Chitosan films are currently available in a non?loaded form used as a haemostatic device. Incorporating vancomycin such these degradable films could offer a potential aid in the treatment of musculoskeletal infections. Future studies for this approach to localized drug delivery include further characterization of release kinetics ! as well as evaluation in a contaminated goat model to test the in vivo efficacy of these films against P. aeuroginosa. The results presented in this study offer insight to the potential use of degradable chitosan films as a carrier vehicle for antibiotics in musculoskeletal applications.
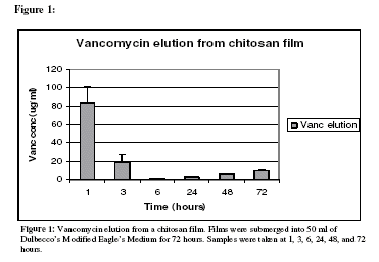
Table 1
| Table 1.Elution data from amikacin and vancomycin loaded films(mean values±standard deviation) | ||||||
|---|---|---|---|---|---|---|
| Antibiotic | 1 hour | 3 hours | 6 hours | 24 hours | 48 hours | 72 hours |
| Amikacin(µg/ml) | 50.69±4.49 | 2.15±1.13 | 3.17±1.14 | 3.99±0.57 | 4.7±2.49 | 0.6±0.48 |
| Vancomycin(µg/ml) | 83.45±17.25 | 19.35±7.98 | 0.58±0.98 | 2.59±0.66 | 9.72±1.92 | 6.19±0.39 |
